Program 2. Axonal transport of endo-lysosomal organelles and presynaptic cargos in the maintenance of axon cellular homeostasis and synaptic function
Goals and Objectives:
Objective One. Axonal transport of autophagy-lysosomal organelles for the maintenance of distal degradation capacity and homeostasis in healthy and diseased neurons.
Neurons are highly polarized cells with an extremely long axon, and thus face exceptional challenges in maintaining distal cellular homeostasis and synaptic function. Lysosomes serve as degradation hubs for autophagic and endocytic components. Endocytic and autophagic organelles generated in distal axons are transported retrogradely to the cell body where mature lysosomes are relatively enriched. However, lysosomes are also recruited to distal axons to achieve local degradation capacity. Therefore, bi-directional transport of these degradative organelles plays a critical role in the maintenance of axonal and synaptic homeostasis and function. Autophagy-lysosomal dysfunction contributes to the pathogenesis of major neurodegenerative diseases and lysosomal storage disorders (LSDs). However, mechanistic contributions of impaired axonal trafficking and dysfunction of lysosomes to disease onset and progression remain largely elusive. Our central hypothesis is that neurons coordinate axonal bi-directional transport of autophagic-lysosomal organelles for the maintenance of distal degradative capacity; transport defects lead to autophagic stress, axonal degeneration, and synaptic dysfunction. Specific aims are formulated to address three fundamental issues: (aim 1) how neurons recruit active mature lysosomes into distal axons to eliminate protein aggregates and damaged organelles; (aim 2) how elevated cholesterol compromises lysosomal function and trafficking in LSD neurons, thus leading to autophagic stress and axonal dystrophy; how the pharmacologic reduction of lysosomal membranous cholesterol reverses disease phenotypes; and (aim 3) how impaired endolysosome transport in dopaminergic neurons contributes to Parkinson’s Disease-linked axonal degeneration.
Objective Two. Axonal transport mechanisms underlying neurodevelopmental disorders.
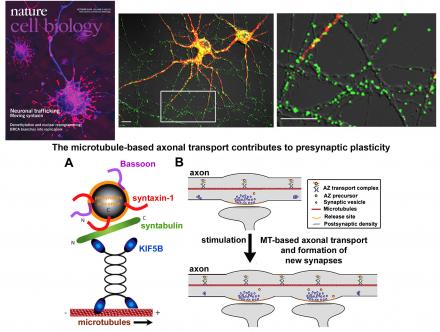
Synapses are distantly located from the cell bod, and thus the formation of new synapses and the maintenance and remodeling of mature synapses require seamless integration of axonal transport of presynaptic cargos. Among these presynaptic components is the scaffolding protein Bassoon, which functions as an organizer of the active zone (AZ) and appears first at newly formed presynaptic terminals. Bassoon undergoes axonal transport in organelles containing multiple presynaptic components, thus ensuring assembly, formation and maintenance of presynaptic terminals. However, the fundamental question of whether impaired axonal transport contributes to presynaptic pathology inAutism Spectrum Disorders (ASDs), a group of childhood-onset neurodevelopmental disorders, remains largely unaddressed. De novo missense mutations underlie a substantial fraction of risk for developing ASDs. While postsynaptic mechanisms play an important role in the susceptibility to ASDs, it remains unknown whether altered axonal transport of presynaptic cargos, and thus reduced formation, maturation, and maintenance of presynaptic terminals, contributes to ASD-linked pathogenesis. Investigations into axonal transport mechanisms in an in vivomodel system will provide the essential information necessary to identify core presynaptic defects at the onset of ASDs.
Summary of Research
Our primary goal is to elucidate mechanisms regulating axonal transport of various membrane organelles. By employing live imaging of adult neurons from genetic mouse models combined with gene rescue experiments, we have made the following important discoveries.
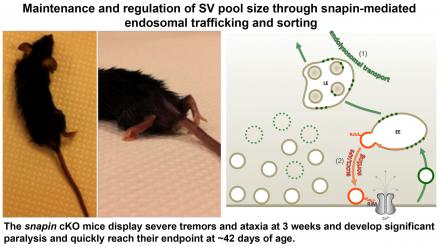
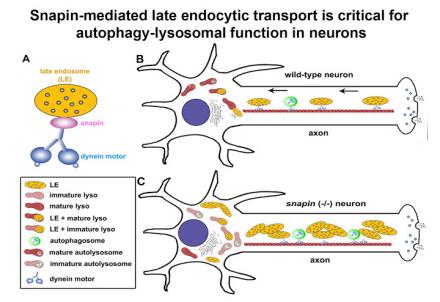
(1) We revealed that snapin acts as an adaptor linking dynein motors to endo-lysosomes and drives their retrograde transport from distal axons to the soma, thus maintaining distal degradation capacity (Cai et al., Neuron 2010).
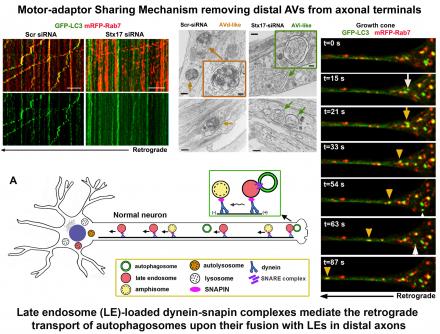
(2) We also revealed a motor-adaptor sharing model by which late endosome-loaded dynein-snapin complex drives the retrograde transport of autophagosomes upon their fusion into hybrid organelles named amphisomes, thus maintaining effective autophagic clearance in distal axons (Cheng et al., JCB 2015).
(3) We demonstrated that the endo-lysosomal pathway exerts a bipartite regulation on presynaptic activity: while endosomal transport influences synaptic vesicle (SV) pool size by shuttling them towards degradation pathways, endosomal sorting determines SV positional priming at release sites (Di Giovanni et al., EMBO J, 2015).
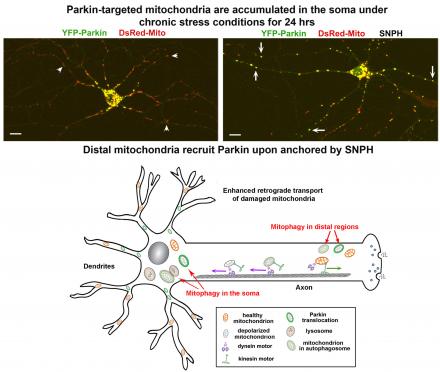
(4) We investigated a familial Amyotrophic Lateral Sclerosis (fALS)-linked mouse model and provided in vitro and in vivo evidence that progressive lysosomal deficits are early fALS-linked pathological events in motor neurons (Xie and Zhou et al., Neuron 2015).
(5) We provided the guidelines for labeling degradative lysosomes in in vitro and in vivo nervous systems to characterize how lysosomal distribution, trafficking, and functionality contribute to neuronal health and disease progression (Cheng et al., JCB 2018).
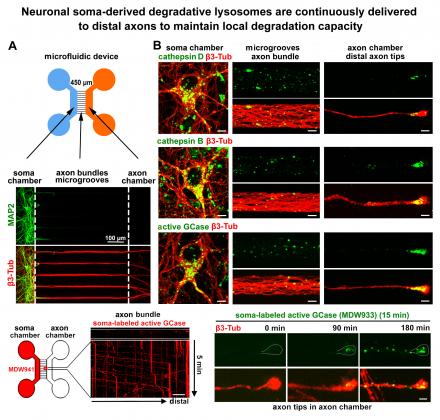
(6) We further demonstrated that degradative lysosomes dynamically transport to distal axons in developing and mature neurons; disrupting axonal lysosome delivery induces autophagic stress. Thus, axonal degradation capacity is maintained through the delivery of “fresh” degradative lysosomes from the lysosomal reservoir in the soma (Farfel-Becker et al., Cell Reports 2019).
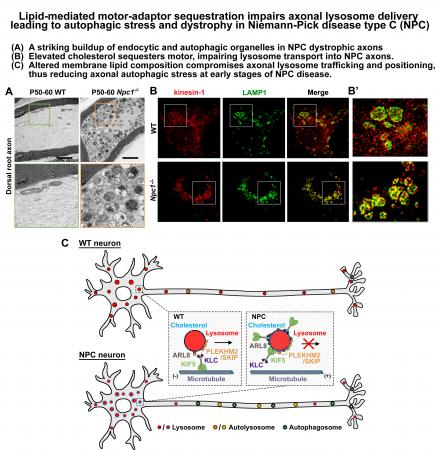
(7) Niemann-Pick disease type C (NPC) is a neurodegenerative lysosomal storage disorder characterized by lipid accumulation in endolysosomes. An early pathologic hallmark is axonal dystrophy occurring at presymptomatic stages in NPC mice. We recently demonstrated a pathological mechanism by which elevated cholesterol on NPC lysosome membranes sequesters motor-adaptor, resulting in impaired lysosome transport into axons, thus contributing to axonal autophagosome accumulation (Roney et al., Developmental Cell 2021).
(8) We identified syntabulin (STB) as a kinesin-1 adaptor that links the motor to presynaptic cargos, thus contributing to presynaptic assembly, maintanence, and plasticity (Su and Cai et al., Nature Cell Biology, 2004; Cai et al., JCB, 2005; Cai et al., Journal of Neuroscience, 2007).
(9) We also revealed that defective axonal transport impairs presynaptic formation and maintenance, thus providing one of the core presynaptic mechanisms underlying autism-like synaptic dysfunction and altered social interactions and communication (Xiong et al., Molecular Psychiatry 2020).