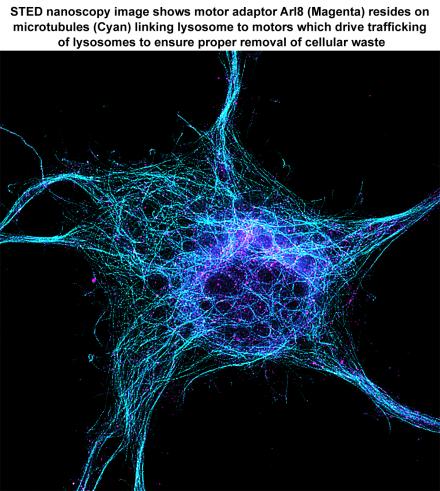
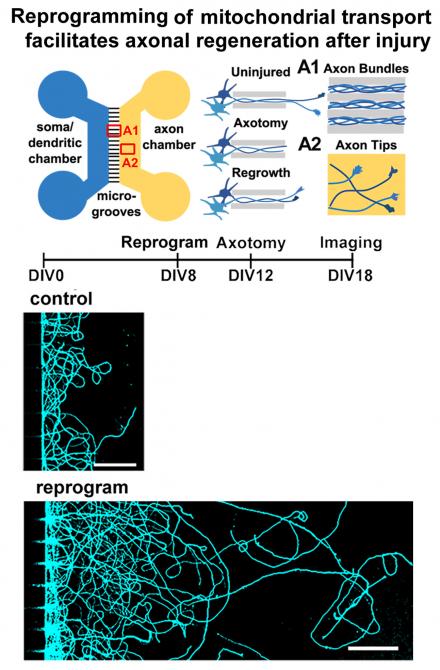
Axonal organelle transport and energy metabolism in neurodegeneration and regeneration
Zu-Hang Sheng, Ph.D
Senior investigator, Chief of Synaptic Functions Section, NINDS, NIH
Dr. Sheng received his Ph.D. in Biochemistry from the University of Pennsylvania in 1993. He completed his postdoctoral research with William Catterall at the University of Washington in 1996. He joined NINDS as an investigator in 1997 and is now a tenured senior investigator and Chief of Synaptic Functions Section at National Institute of Neurological Disorders and Stroke (NINDS), NIH. Dr. Sheng lab has published a number of important studies focused on axonal transport of mitochondria and endolysosomes in healthy and diseased neurons. The lab has used a broad range of approaches to tackle these problems, notably the development of neuronal cultures from adult disease mouse models and live imaging of various organelle transport. He has served as a mentor for 8 graduate students (NIH joint PhD Programs), 6 HHMI-NIH research scholars, and 20 postdoctoral fellows. Eight former trainees were appointed with faculty positions in an academic setting. Dr. Sheng has also served on the editorial board of Journal of Cell Biology (JCB) and Journal of Biological Chemistry (JBC) and associate editor of Autophagy. Dr. Sheng was elected to AAAS Fellow in 2016 and ASCB fellow in 2017 for his contributions to the field of axonal transport of mitochondria and endolysosomes in maintenance of neuronal homeostasis and synaptic function in health and diseases. Dr. Sheng received the 2021 Dr. Francisco S. Sy Award for Excellence in Mentorship at HHS. Dr. Sheng is also the recipient of the 2023 NIH Director’s Award for seminal contributions to the understanding of axonal mitochondrial and lysosomal transport and maintenance of bioenergetics and cellular homeostasis in synaptic transmission and neural regeneration.
Mechanisms regulating axonal organelle transport in healthy and diseased neurons
Sheng laboratory focuses on mechanisms regulating axonal transport that is essential for the maintenance of synaptic function and axonal homeostasis. Using genetic mouse models, his group is addressing several fundamental questions: (1) how mitochondrial transport is regulated to sense changes in synaptic activity, mitochondrial integrity, axon injury and pathological stress; (2) how neurons coordinate late endocytic transport and autophagy-lysosomal function to maintain cellular homeostasis and distal degradation capacity; (3) how impaired transport contributes to synaptic dysfunction and axonal pathology in neurodegenerative diseases. These studies have led to the identification of three motor adaptor and anchoring proteins (syntaphilin, Snapin, and syntabulin) that regulate axonal transport of mitochondria, endo-lysosomes, autophagosomes, and synaptic cargoes. The long-term goal of the laboratory is to decipher mechanisms (1) boosting axonal and synaptic energy metabolism and (2) enhancing autophagy-lysosomal function for efficient clearance of dysfunctional organelles and aggregated proteins that are associated with major neurodegenerative diseases. Pursuing these investigations will advance our knowledge of fundamental processes that may affect human neurological disorders.
|
Video file
|
Image
 |