
The mission of the Clinical Trials Unit (CTU) is to create an environment for state-of-the-art clinical trials to promote integration into the greater translational development of treatments of neurological disease.
As a core unit under the NINDS Office of the Clinical Director, the CTU aims to support and facilitate clinical research at the NINDS intramural research program, by providing assistance and resources during all phases of the life cycle of a protocol, from protocol development, to start-up and execution, to data analysis and reporting.
It is the goal of the NINDS CTU to ensure quality of practice in the NINDS intramural research programs through oversight of clinical research conducted by NINDS intramural investigators and the provision of education and training opportunities to all members of clinical research study teams.
The NINDS CTU is furthermore dedicated to promoting the advancement of clinical trial methodology through research collaborations with clinical investigators at NINDS as well as outside partners.
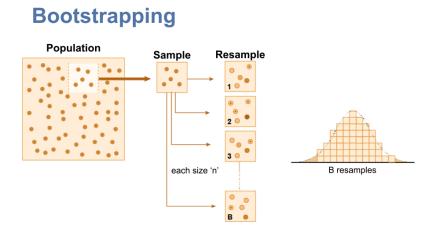
Bootstrapping is a statistical approach that can be used to describe inference of a sample about a population. We can use this to create simulated datasets with characteristics similar to our original dataset for the purposes of estimation or describing variability. This procedure can be done by sampling “with replacement” from the original dataset, to create many (perhaps thousands) of new simulated datasets of the same size n. From each sample, we can extract some summary statistic, such as a mean, to get an understanding of the possible distribution of means we can get from similar datasets.
Who should be listed on the FDA Form 1572?
A sub-investigator, per FDA, includes any individual member of the research team who assists the investigator and makes a direct and significant contribution to the data. In general, if an individual is directly involved in the performance of study-specific procedures that make a significant contribution to the data and/or the collection of data, that person should be listed on the 1572. Anyone who documents results or findings on a study-specific form always should be listed on the Form 1572.
