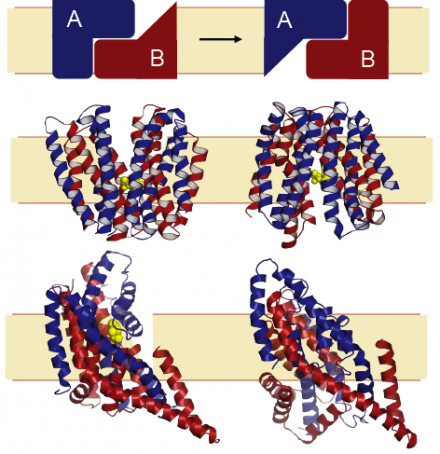
Membrane-embedded proteins are essential components of cellular organisms, allowing cells to communicate with their surroundings by providing bridges through the barrier that the lipid membrane forms. Our group is interested in understanding the mechanisms of membrane proteins using computational and theoretical approaches. Of particular interest are transporter proteins, which capture the chemical potential energy of ionic gradients (across the membrane) to facilitate movement of essential chemicals, or unwelcome toxic compounds, into and out of the cell. A fundamental question is how transporters achieve the required levels of specificity for a given chemical, or substrate, and how the protein-substrate interaction is coupled to transport of ions such as sodium. A further puzzle is how the transporter undergoes the requisite changes in shape to allow access of the substrate binding sites to either side of the membrane, while also preventing leakage (see Figure).
An essential characteristic of our work is that the hypotheses and interpretations we provide be connected with experimental evidence. Close and long-standing collaborators include the Rudnick group at Yale University, with whom we have studied transport of neurotransmitters such as serotonin. We also study glutamate transport in collaboration with the Kanner group, and acetylcholine transport with the Schuldiner lab, both from Hebrew University, Jerusalem. An ongoing collaboration with the Ziegler group at MPI Frankfurt/University of Regensburg combines computational, structural and biochemical studies on an osmolyte transporter. An aspect we find particularly fascinating is the role of repeated elements in the mechanisms of transport conformational change, which provide an elegant pseudo-symmetry and degeneracy to the system so that a single protein can adopt two pseudo-symmetric states (see Figure).
We apply a range of computational tools to understand these processes at atomic detail. To date, those techniques include sequence analysis and continuum electrostatics, as well as protein structure prediction and molecular dynamics simulation, tools for which we also develop ourselves.
The Pseudo-Symmetry of Transporter Structural Changes: