
BG 10 RM 7D41
10 CENTER DR
BETHESDA MD 20814
Dr. Wassermann received his B.A. from Swarthmore College, his M.A. from the University of Pennsylvania, and his M.D. from New York Medical College. After neurology residency at the Boston City Hospital, he came to the NINDS Human Motor Control Section as a postdoc, to study motor cortex physiology and the control of voluntary movement. As a fellow, he pioneered many of the fundamental techniques of transcranial magnetic stimulation (TMS) and collaborated on the first clinical use of TMS in the treatment of depression. Dr. Wassermann established an independent laboratory in 1996 and has focused on using noninvasive stimulation techniques combined with functional neuroimaging to measure and influence plastic processes in the human brain.
We study the brain systems underlying learning, memory and sensorimotor adaptation, using noninvasive brain stimulation, functional and structural neuroimaging, and innovative behavioral paradigms. The main thrust of this basic human research is how to make learning and adaptive brain plasticity more efficient in patients with brain disorders and healthy people. Our current work is focused on how changes in activity and connectivity in brain networks code the unpleasantness of pain and physical effort. We are also using TMS to modulate activity in this network in the hope of reducing disability in disorders of pain and fatigue.
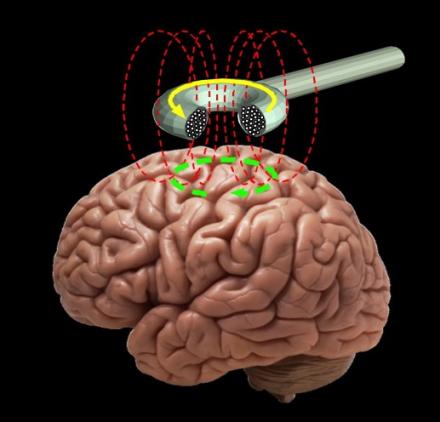
We have been involved used transcranial brain stimulation as a research tool since 1989 and have performed some of the key studies to validate the techniques and establish guidelines for their safe use. Transcranial magnetic stimulation is a noninvasive means of getting electrical energy across the insulating tissues of the head and into the brain. A powerful and rapidly changing electrical current is passed through a coil of wire applied near the head. The magnetic field, oriented perpendicular to the plane of the coil passes virtually unimpeded through the scalp and skull. In the brain, the magnetic field produces currents in the induced electrical field lying parallel to the plane of the coil. These currents are able to excite neural processes lying in the plane of the induced field in a manner roughly analogous to direct cortical stimulation with electrodes. In properly designed experiments, TMS can be a powerful physiological probe of cortical cortical function for clinical and basic neurophysiology. It is also an effective technique for altering the responsiveness of human brain circuits and may have therapeutic applications, as well. One of our aims is to promote reproducible research in this area and facilitate the transfer of transcranial stimulation techniques from the laboratory to the clinic. .
Clinical Protocols:
-
Effects of prism adaptation and rTMS on brain connectivity and visual representation 16-N-0170
-
Exploring the relationship between brain asymmetry and attention 19-N-0036
-
Modulating the hippocampal and striatal memory circuits with TMS 19-N-0114
-
Task-dependent effects of TMS on the neural biomarkers of episodic memory 000196
Selene Schintu, PhD
Kristen Warren, PhD
Nicholas Madian PhD
Hedyeh Bagherzadeh Omandani PhD
Kofi Boateng, BS
Freedberg M, Cunningham CA, Fioriti CM, Murillo JD, Reeves JA, Taylor PA, Sarlls JE, Wassermann EM. (2021). Multiple parietal pathways are associated with rTMS-induced hippocampal network enhancement and episodic memory changes. Neuroimage, 118199. https://doi.org/10.1016/j.neuroimage.2021.118199
Freedberg MV, Reeves JA, Fioriti CM, Murillo J, Voss JL, Wassermann EM (2022) A Direct Test of Competitive Versus Cooperative Episodic-Procedural Network Dynamics in Human Memory. Cereb Cortex. 2022.
Freedberg MV, Reeves JA, Fioriti CM, Murillo J, Wassermann EM (2022) Reproducing the effect of hippocampal network-targeted transcranial magnetic stimulation on episodic memory. Behav Brain Res 419:113707.
Schintu S, Gotts SJ, Freedberg M, Shomstein S, Wassermann EM (2022a) Effective connectivity underlying neural and behavioral components of prism adaptation. Front Psychol 13:915260.
Schintu S, Kravitz DJ, Silson EH, Cunningham CA, Wassermann EM, Shomstein S (2023) Dynamic changes in spatial representation within the posterior parietal cortex in response to visuomotor adaptation. Cereb Cortex 21; 33: 3651-3663.
Freedberg M, Cunningham CA, Fioriti CM, Murillo JD, Reeves JA, Taylor PA, Sarlls JE, Wassermann EM. (2021). Multiple parietal pathways are associated with rTMS-induced hippocampal network enhancement and episodic memory changes. Neuroimage, 118199.
Schintu S, Cunningham CA, Freedberg M, Taylor P, Gotts SJ, Shomstein S, Wassermann EM. Callosal anisotropy predicts attentional network changes after parietal inhibitory stimulation. Neuroimage. 2021 Feb 1;226:117559. doi: 10.1016/j.neuroimage.2020.117559. Epub 2020 Nov 13. PMID: 33189929; PMCID: PMC7885523.
Schintu S, Freedberg M, Gotts SJ, Cunningham CA, Alam ZM, Shomstein S, Wassermann EM. Prism Adaptation Modulates Connectivity of the Intraparietal Sulcus with Multiple Brain Networks. Cereb Cortex. 2020 Jul 30;30(9):4747-4758. doi: 10.1093/cercor/bhaa032. PMID: 32313949; PMCID: PMC7526755.
Freedberg M, Reeves JA, Hussain SJ, Zaghloul KA, Wassermann EM. Identifying site- and stimulation-specific TMS-evoked EEG potentials using a quantitative cosine similarity metric. PLoS One. 2020 Jan 13;15(1):e0216185. doi: 10.1371/journal.pone.0216185. PMID: 31929531; PMCID: PMC6957143.
Freedberg M, Reeves JA, Toader AC, Hermiller MS, Kim E, Haubenberger D, Cheung YK, Voss JL, Wassermann EM. Optimizing Hippocampal-Cortical Network Modulation via Repetitive Transcranial Magnetic Stimulation: A Dose-Finding Study Using the Continual Reassessment Method. Neuromodulation. 2020 Apr;23(3):366-372. doi: 10.1111/ner.13052. Epub 2019 Oct 30. PMID: 31667947; PMCID: PMC7657658.
Freedberg M, Reeves JA, Toader AC, Hermiller MS, Voss JL, Wassermann EM. Persistent Enhancement of Hippocampal Network Connectivity by Parietal rTMS Is Reproducible. eNeuro. 2019 Oct 16;6(5):ENEURO.0129-19.2019. doi: 10.1523/ENEURO.0129-19.2019. PMID: 31591137; PMCID: PMC6795558.
Schintu S, Freedberg M, Alam ZM, Shomstein S, Wassermann EM. Left-shifting prism adaptation boosts reward-based learning. Cortex. 2018 Dec;109:279-286. doi: 10.1016/j.cortex.2018.09.021. Epub 2018 Oct 12. PMID: 30399479; PMCID: PMC7327780.
