Background
The Proteomics Core Facility provides amino acid sequencing of purified proteins/peptides for NINDS investigators. The facility is also available for collaborations involving protein/peptide purification and more complicated sequencing strategies.
Contact Information
Yan Li, Ph.D., Core Director
Phone: 301-402-1690
E-mail: liy26@ninds.nih.gov
Scope of the Core Facility
The Core provides amino acid sequencing and mass spectral analysis of proteins and peptides for NINDS investigators. The Core also provides theoretical and technical expertise for the separation and purification of proteins and peptides and performs "Proteomics" type projects including the identification of proteins separated by 1- and 2-D PAGE gels and identification of protein phosphorylation sites.
Core Facility Equipment
- Agilent Technologies Model 1100 series HPLC Modular System equipped with refrigerated autosampler, diode array UV detector and refrigerated fraction collector for protein and peptide isolation and purification.
- Applied Biosystems Procise Model 491 pulsed-liquid protein sequencer that performs automated classical Edman degradation.
- Mass Spectrometers: A) ThermoFinnigan ProteomeX HPLC/ELECTROSPRAY IONIZATION MASS SPECTROMETER (HPLC/ESI/MS) system consisting of a Surveyor Capillary HPLC interfaced to a LCQ Deca XP Plus ion trap ESI Mass spectrometer. This combination enables us to perform automated capillary (0.17 mm columns) RP-HPLC separations of proteolytic digests obtained from minute amounts of proteins (30 ng) in SDS PAGE 1&2D gel slices. The system performs on the fly mass spectral MW measurement and MS/MS sequencing of the resulting peptides.
- The system also performs automated 2D HPLC\MS\MS proteomics analysis in which protein digests are first separated by strong cation exchange chromatography (SCX) in the first stage followed by a reversed–phase separation in the second stage of each of the SCX fractions. The 2D HLPC is widely seen as complementary to or a replacement of 2D electrophoresis in proteomics. (B) Matrix Assisted Laser Adsorption Ionization Time-Of-Flight MASS SPECTROMETER (MALDI-TOF MS). Applied Biosystems Voyager DE-STR MALDI-TOF.
- This instrument is capable of extremely high resolution (>30,000 at 12 kDa) and sensitivity for the analysis of peptides and proteins up to 340 kDa. Because of these features in addition to its automation capability, it is the ideal instrument for Large Scale Proteomics projects.
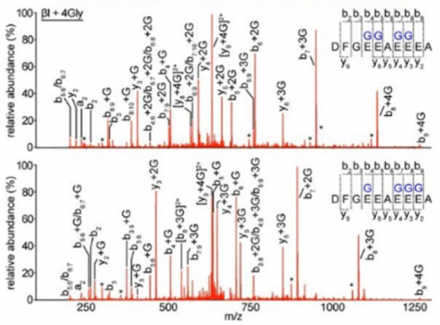
An example of PTM analysis, mapping Glycylation on beta I tubulin.
Selected Publications Using the Core
- Szajner, P., Jaffe, H, Weisberg, A.S. and Moss, B., Vaccinia virus G7L protein interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions, J. Virol., 77(6), 3418-3429, 2003.
- Li, B.S., Ma, W., Jaffe, H., Zheng, Y., Takahashi, S., Zhang, L., Kulkarni, A.B. and Pant, H.C., Cyclin-dependent kinase 5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival, J. Biol. Chem., 278(37), 35702-35709, 2003.
- Reddy, P.T., Prasad, C.R., Reddy, P.H., Reeder, D., McKenney, K., Jaffe, H., Dimitrova, M.N., Ginburg, A., Peterkofsy, A. and Murthy, P.S., Cloning and expression of a gene for a novel protein from Mycobacterium smegmatis with functional similarity to eukaryotic calmodulin, J. Bacteriol., 185(17), 5263-5268, 2003.
- Jaffe, H., Vinade, L. and Dosemeci, A., Phosphoproteins in the postsynaptic density: a proteomic analysis, submitted to Biochem, 2003.
- Sieckmann, D.G., Jaffe, H., Golech, S., Cai, D-G, Hallenbeck, J.M. and McCarron, R.M., Alpha-2-macroglobulin is a growth regulatory protein in plasmas of hibernating 13-lined ground squirrels and woodchucks, submitted to Am. J. Physiol. 2003.
- Das S, Gerwin C. and Sheng, Z.H., Syntaphilin binds to dynamin-1 and inhibits dynamin-dependent endocytosis, J Biol Chem., 278(42): 41221-41226, 2003 (Acknowledgment).