
BG 10 RM B1D733
10 CENTER DR
BETHESDA MD 20814
After receiving the US DOE Alexander Hollander Distinguished Post-doctoral Fellowship at Brookhaven National Laboratory, Dr. Latour became Director of Applied Science at Medical Advances with an appointment in Medical Physics at the Medical College of Wisconsin. He was recruited to NINDS-DIR as a Staff Scientist in 2000 to help build the Section on Stroke Diagnostics and Therapeutics. The branch conducts clinical research at the Stroke Centers of Suburban Hospital in Bethesda, Maryland and Washington Hospital Center in the District of Columbia, where the NINDS has placed stroke teams and MRI research facilities. For ten years, he was the director of the DOD funded Center for Neuroscience and Regenerative Medicine Acute Studies Core leading a team studying acute TBI in civilians. In 2016 he became the PI of the NINDS Acute Cerebrovascular Diagnostics Unit. His research interests focus on the use of MRI to better identify, classify, and treat acute stroke and brain injury.
The Acute Cerebrovascular Diagnostics Unit (ACDU) is part of the National Institute of Neurological Disorders and Stroke. The foundation of our lab is an acute clinical service that allows rapid identification, screening, recruitment, retention, and referral, to study the early events in cerebral ischemia in acute stroke and traumatic brain injury (TBI). The research in the program focuses on the use of magnetic resonance imaging (MRI) to identify markers coupled to the biology of the acute cerebrovascular injury and the response to therapy.
Overview
While we have a physical presence on the NIH campus in Bethesda, MD, our clinical research is primarily based at two Washington DC metropolitan area hospitals, where we provide care for approximately one thousand stroke patients per year. The complexity of clinical care is humbling and the chasm between bench research and the application to patients is difficult to span. Immediate and unfettered access to MRI is a hallmark of the program, allowing imaging to be performed in the first hour after presentation and immediately after acute intervention, often in critically ill patients. Our MR imaging aids the team daily in their care of patients, while novel observations made on MRI lead to discovery of and deepen our comprehension of relevant pathophysiology, and ultimately improve outcomes through more precise stratification and targeted treatment. Thus, we are bridging the translational gap through non-invasive MR imaging and multi-disciplinary science.
Traumatic Brain Injury
Through support of the US Department of Defense (DoD) sponsored, congressionally mandated collaborative, the Center for Neuroscience and Regeneration, Dr. Latour assembled a new team, partnered with NINDS stroke-team hospitals and began a program to study acute head injury. The broad objective was to identify early and longitudinal markers on MRI specific to TBI that are predictive of clinical outcomes. Over the course of ten years, we enrolled nearly two-thousand patients across two protocols. Our study approach is intentionally broad and evokes multi-disciplinary science, including MRI targeted histology, blood-based biomarkers and clinical trials as these collaborations are critical to further the investigation of acute brain injury.
TBI shares many pathologic features of stroke, including cerebral ischemia, blood–brain barrier disruption, acute hemorrhage, the innate and adaptive immune response, and related secondary injury. Due to the intrinsic approach of our program and with the support of the DoD, we have discovered a novel finding suggestive of meningeal injury, identified the pathophysiological correlate of traumatic microbleeds via MRI targeted histology, and now conjecture meningeal-vascular injury is one of the predominant injury mechanisms in mild TBI.
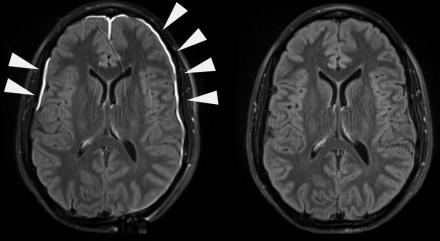
Traumatic Meningeal Injury (TME)
In traumatic brain injury (TBI), traumatic vascular injury may occur in the brain parenchyma (intraparenchymal hemorrhage or microbleeds) or in vessels traversing the meninges (subdural hematoma or subarachnoid hemorrhage). The meninges serve as a functional barrier surrounding the brain, critical to the immune response, and can be compromised following head trauma. Following head trauma, gadolinium-based contrast agent used in MRI extravasates from the vasculature, enhancing the injured dura within minutes. The contrast agent later permeates the subarachnoid space in some patients analogous to the blood brain barrier disruption detected in acute ischemic stroke. Studies elucidating the biology of the brain’s immune response implicate disruption of these barriers as contributors to the neuronal and cognitive dysfunction that occurs in normal aging, vascular cognitive impairment, Alzheimer’s disease, multiple sclerosis, and other neurodegenerative disorders.
Traumatic microbleeds (TMBs)
Acute traumatic microbleeds conspicuously appear on MRI after TBI even in patients without findings on CT. TMBs have a high prevalence in acute TBI, predominantly in our mild population, and we found that those with evidence of TMBs were twice as likely to have disability at 30- or 90-days post-injury. After co-localizing TMBs seen on in vivo MRI and post-mortem high-resolution 7T MRI to histopathology, we saw iron-laden macrophages in the perivascular space surrounding injured vasculature, suggesting TMBs are a form of traumatic vascular injury. The presence of TMBs may be a biomarker for vascular related interventions after TBI, as the leakage of blood from damaged blood vessels can trigger an inflammatory response. Thus, we postulate that TMBs may be useful biomarkers for identifying TBI patients that may benefit in the acute phase from therapies aimed at minimizing acute ischemic damage, or in the chronic phase, at improving microvascular cerebral blood flow.
Stroke
Significant advances have been made in our understanding of acute ischemic stroke and therapeutic interventions in the program over the past two decades. Much of our research has focused on observations on MRI obtained immediately after the patient presents to the hospital with stroke-like symptoms. Despite these significant advances, acute therapy for ischemic stroke remains largely limited to reperfusion monotherapy in a fairly select population. We are studying the use of MRI to rapidly identify patients presenting to the emergency department with minor, potentially non-disabling deficits as a mechanism for expanding access to acute therapy. Relatively recently, endovascular therapy (EVT) through mechanical embolectomy, a minimally invasive technique used to remove the clot under angiographic guidance, has been shown to be highly efficacious. Despite surgical success, a large proportion of patients do not fully recover. This presents an opportunity to use MRI to improve our understanding of why.
GUARDS (Guiding neUroprotection After Reperfusion to prevent Damage in acute ischemic Stroke)
We are using MRI obtained early after EVT induced reperfusion to study secondary injury mechanisms. Thrombectomy is successful and blood flow is rapidly restored in most patients undergoing therapy. Ideally, blood flow normalizes in the tissue and the ischemic cascade is halted. However, in some patients, injury progresses in the form of blood-brain barrier disruption, acute cerebral edema, hemorrhagic transformation, and acute and chronic inflammation. We are investigating novel markers to better stratify these injury mechanisms and identify new therapeutic strategies to ameliorate secondary damage.
TIMES (Treatment of mInor stroke with MRI Evaluation Study)
In many patients, symptoms of acute stroke are minor, fluctuating, resolving, or not specific to stroke. A proportion of these patients go on to more severe symptoms and disability. Often the CT scan obtained in the emergency department is negative leaving the patient with uncertain diagnosis. However, there remain equipoise within the field as to the risk/benefit of treating with tissue plasminogen activator (rtPA). We have found that MRI can be used to identify patients with acute imaging findings consistent with acute stroke and are exploring strategies for acute intervention.
Automated imaging platform
Through a collaboration with NIH-CIT using Mipav, our group has provided an automated MR imaging platform for ischemic stroke diagnosis in real-time at our hospitals. The fully automated algorithm, “coretool”, calculates core based on a novel heuristic approach using combined imaging characteristics to detect and quantify the presence of acute ischemia. We have expanded this automated platform to include perfusion imaging to estimate the amount of treatable penumbra in real-time. Another ongoing development is to incorporate machine learning for automated hemorrhagic transformation detection following acute intervention.
